Abstract
Background: TNB-486 is a novel CD19xCD3 bispecific T-cell engager (TCE) that incorporates a unique anti-CD3 moiety designed to reduce cytokine release syndrome by binding to T-cells with low affinity. A silenced IgG4 backbone confers a long half-life suitable for intermittent administration. A combination of TNB-486, which targets CD19, with R-CHOP, which incorporates CD20 targeting via Rituxan, in DLBCL may lead to synergistic tumor kill and reduce the risk of antigen escape, offering a promising approach for improved long-term remission. Here, interim results of the ongoing FIH phase 1 study of TNB-486 in R/R B-NHL are presented (NCT04594642).
Methods: The primary objectives of the study were to assess the safety, tolerability and pharmacokinetics of TNB-486 when administered as monotherapy and to determine the optimal biologically active dose. Patients with R/R B-NHL after at least 2 prior lines of therapy were eligible; prior anti-CD19 therapy was permitted. Patients received escalating doses of TNB-486 infused IV over 1-2 hours Q2W until PD/unacceptable toxicity. Fixed doses were given initially at lower doses, followed by a priming dose at higher target doses (>2.4 mg). Responses were assessed by RECIL 2017 and adverse events were graded using CTCAE v5.0, except for cytokine release syndrome (CRS) and neurotoxicity (NT) which were graded according to ASTCT criteria.
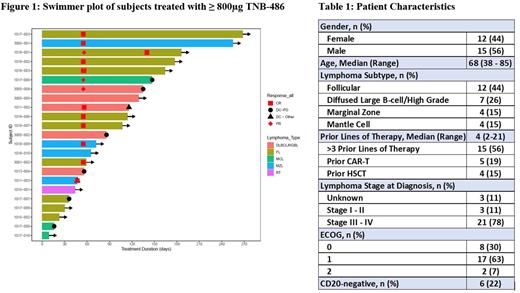
Results: As of 01 July 2022, 27 subjects have received TNB-486 (0.030 - 10 mg). 22 patients were evaluable for efficacy and 27 for safety. Patient characteristics are summarized in Table 1. Overall, patients were heavily pre-treated, with a median of 4 prior lines of therapy (ranging from 2 - 21 prior lines). 19% of enrolled subjects had previously failed CAR-T.
The most common Gr3/4 AEs were Lymphopenia (26%) and Neutropenia (15%). The most common related AEs were CRS (59%), Neutropenia (30%), Lymphopenia (26%) and Anemia (26%). No Gr ≥ 3 CRS was observed, and no CRS (any grade) occurred post-cycle 1. Median time to onset of CRS was 1 day with a median time to resolution of 1.5 days (ranging from <1 - 9). Eight subjects received tocilizumab; six of these had Gr2 CRS. Six subjects (22%) experienced NT (Grade 3 7%, no Grade 4, occurring in two subjects with MZL and Richter's transformation, respectively), all were transient and resolved without sequelae. No Gr2 or higher NT was reported in DLBCL or FL, and no new case of NT occurred beyond cycle 1. Four subjects received steroids for NT. Notably, 1 subject experiencing Gr3 NT continued therapy at a reduced dose with no recurrence.
No fatal events occurred. Three DLTs were observed, including two Gr3 NT events and one Gr4 thrombocytopenia, all of which resolved without sequelae. Only 1 subject out of 27 discontinued treatment due to a TNB-486 related AE (Gr 3 NT); this subject remains in CR 4 months post-last-dose.
Preliminary PK data showed an average T1/2 that ranged from 7.55 - 11.7 days at active doses, supporting the Q2W (or less frequent) dosing of TNB-486.
The overall response rate (ORR) at ≥ 800µg was 72% (13/18 evaluable subjects), with a CR rate of 61% (11/18). Among response-evaluable CAR-T exposed subjects, 2/3 achieved a CR. Among response-evaluable FL subjects treated at ≥ 800µg, the ORR was 88% (7/8 subjects); all responding subjects achieved a CR. Five DLBCL subjects were treated at ≥ 800µg with an ORR of 40%; one subject achieved an MRD-negative CR. Notably, this subject had been previously treated with 5 lines of therapy including CAR-T and never achieved a CR prior to this study. Among 4 subjects with MZL, the ORR was 75% (all CR). At a median follow-up of 3.8 months (range: 1.5 - 8.7 months), no subjects in CR have relapsed, with ongoing remission at up to 10 months post-initiation of TNB-486 therapy (Figure 1). Responses were seen across all NHL subtypes and prognostic factors (e.g. disease burden, prior lines of therapy, and refractoriness to prior therapy).
Conclusions: Preliminary data from the current FIH study of TNB-486 show a tolerable safety profile with mostly low-grade CRS/NT at doses up to 7.2 mg (dose escalation ongoing) and promising activity in heavily pre-treated B-NHL. CD19 targeting with TNB-486 offers the opportunity to combine with CD20-directed therapies to maximize tumor targeting and achieve deep and durable remissions in the frontline as well as the relapsed/refractory setting.
Disclosures
Jacobs:Beigene: Speakers Bureau; TG Therapeutics: Research Funding; Verastem: Consultancy; Janssen: Speakers Bureau; Teneobio: Research Funding; MEI Pharma: Research Funding; AstraZeneca: Consultancy, Speakers Bureau; Pharmacyclics, an AbbAvie Company: Consultancy, Research Funding, Speakers Bureau; AbbVie: Consultancy, Speakers Bureau. Devata:Astra Zeneca: Current equity holder in publicly-traded company; Bayer: Current equity holder in publicly-traded company; GSK: Current equity holder in publicly-traded company; Novo Nordisc: Current equity holder in publicly-traded company; Abbvie: Current equity holder in publicly-traded company; Bristol Myers Squibb: Current equity holder in publicly-traded company; Johnson & Johnson: Current equity holder in publicly-traded company; Lilly Eli: Current equity holder in publicly-traded company; Merck: Current equity holder in publicly-traded company. Gaballa:ADC Therapeutics: Speakers Bureau; Abbvie: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; Beigene: Consultancy; Epizyme: Consultancy, Other: research support. Yoon:GSK: Honoraria; Boryung: Honoraria, Research Funding; Celltrion: Honoraria, Research Funding; Novartis: Honoraria; BMS: Honoraria; Amgen: Honoraria; Beigene: Research Funding; Sanofi: Research Funding; Samyang: Honoraria, Research Funding; Takeda: Honoraria; GI Cell: Consultancy; Ab Clone: Consultancy; Pharos iBio: Consultancy; Janssen: Honoraria, Research Funding; Kirin: Honoraria, Research Funding; Roche: Honoraria. Buelow:Teneobio: Ended employment in the past 24 months; Ancora Biotech: Current Employment, Current equity holder in private company. Nair:Incyte Corporation: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal